It seems likely that all relevant groups are cowards, and none are willing to move forward without a more favorable political context. But there's another possibility not considered here: perhaps someone has already done a gene-drive mosquito release in secret, but we don't know about it because it didn't work. This might happen if local mosquito populations mix too slowly compared to how long it takes a gene-driven population to crash; or if the initially group all died out before they could mate; or something in the biology of the driven-drive machinery didn't function as expected.
If that were the situation, then the world would have a different problem than the one we think it has: inability to share information about what the obstacle was and debug the solution.
Is there an actually good argument for why eliminating only disease carrying mosquitoes is acceptable, rather than just wiping them all out? There is no question that even without the threat of malaria, creatures like mosquitoes, bed-bugs and horse-flies decrease the quality of life of humans and animals. Would the effects on ecosystems really be so grave that they might plausibly outweigh the enormous benefits of their extinction?
The returns from just removing mosquitoes because their sting is annoying are tiny compared to eradicating disease carrying ones, and mosquitoes are pollinators. There are real risks to just eliminating them all at a whim, plus honestly it's just not a habit we should get into.
To me it feels exactly like the kind of habit we should get into.
Imagine an advanced (possibly alien) civilization, with technology far beyond ours. Do you imagine its members being pestered by bloodsucking parasites? Me neither.
The existence of mosquitoes is an indictment of humanity, as far as I'm concerned.
Imagine an advanced (possibly alien) civilization, with technology far beyond ours. Do you imagine its members being pestered by bloodsucking parasites?
Maybe not, but that says little about what path they take to avoid that. Maybe they simply have perfect repellents, or live inside extremely well-controlled habitats, or have gene-engineered themselves to not be tasty (some of us aren't! I hardly ever get stung, my wife gets stung all the time). I feel like if you're advanced enough, the safest and most efficient path tends to be that of fine control and minimum disruption rather than steamrolling your way through the world. Consider how surgery evolved for example from crude near-butchery to using optic fibers and tiny robotic probes to perform operations with a light touch in a lot of cases - and surely you could imagine your advanced civilization doing even better, with no need to cut any flesh at all in most cases. And there is an order to things - you would not want to (nor would benefit from) start producing hydrofluoric acid before you have plastic vessels to contain it in. My impression is that anyone willing to do something as crude and destructive as driving an entire family of animals to extinction just to avoid some itchy stings probably doesn't have the knowledge to do so safely, and anyone who would have that knowledge would probably know a thousand better ways to do the same thing.
Old fashioned lobbying might work. Could there be a political candidate in a relevant country that could build a strong platform on getting rid of malaria?
I am very in favor of gene drives for eliminating or altering disease carrying pests, and upset that society is irrational enough not to have seized the opportunity. I have heard of people trying to talk to locals about this, and having trouble with communicating the ideas and getting approval. I wonder if it might be worth exploring a more subtle change rather than a gene drive focused on infertility?
For instance, the plan to modify the mosquitos so that they are resistant to becoming carriers of malaria. If this was done in such a way as to give the mosquitos receiving this gene also a resistance to a particular pesticide, then you could spread the pesticide (and not other pesticides) in the region, selecting for the non-malaria-carrying mosquitos. Then you just have to consider the risk of malaria itself becoming resistant to the anti-malaria mosquito genes, as your footnote mentions. The best way to prevent this would to have the effort be done thoroughly enough across the world to cause malaria to go extinct without having a chance to develop resistance. And preparing multiple different methods of making mosquitos resistant, so that the malaria would have to have multiple lucky mutations to get around it rather than just one.
I think it's worth exploring to see if a plan like this was an easier sell, politically.
It depends on whether the main political objection is that "GMO's are scary" or that "killing mosquitoes will disrupt ecosystems". (I've heard both objections, but strongly disagree with them).
If you made malaria-resistant mosquitoes, you'd actually have GMO mosquitoes biting people. With a gene drive that prevents female mosquito development, no transgenic mosquitoes would bite people since they would all be male.
Who is making the "GMOs are scary" and "killing mosquitoes will disrupt ecosystems" argument? The African public? African government officials? Or Westerners who have no skin in the game? If the latter, then why should we care about what they think? The important thing is public sentiment in Africa. If that goes well, then things should be fine.
Consider, for instance, George W. Bush. A fairly mixed reputation in the West, reviled in the Middle-East and beloved by Africans. Wait, what? Bush championed an anti-Aids initiative in Africa which saved the lives of millions. Westerner's, and people form the Middle East don't hear about this, but the people whom the initiative affected sure do and they approve.
then you could spread the pesticide (and not other pesticides) in the region
This would affect other insects in addition to the targeted mosquitoes, right? This seems strictly worse than the original gene drive proposition to me.
You could use a more targeted pesticide. Also, people are already spraying pesticides to kill disease carrying mosquitos. I'm not saying it's a better option from a scientific/engineering/logic perspective, just that it should be something to do market research on to see if the target populations are less irrationally creeped out by it.
A simple plan of:
>Get $10 million from EAs
>Bribe an African minister for release approval
>Acquire gene drive mosquitos from existing research programs by asking nicely
>Release and monitor
should be sufficient.
The whole gene drive mosquito thing has slipped my mind, but I am once again reminded that this is my calling. The last time I ignored/half-assed a gut feeling like this, I missed out on becoming a Bitcoin millionaire.
Never again.
What progress have you made since last year? Have you made any contacts and reached out directly to anyone in the field?
I strongly disagree with bribery, I think it has the potential to cause a huge scandal which is exactly what we don't want here.
I meant the standard "development aid" that is then quickly embezzled by whatever minister can actually get things done. Remember that they are taking a major risk if something goes wrong. Or everything goes right but some Western Eco NGO gets upset and talks to their NGO buddies resulting in a funding reduction.
The main reason that there hasn't been a single jurisdiction that was willing to agree to field releases is that no one has been trying hard enough. If you offer enough money, you can find a decision-maker somewhere who is willing to let you release a few mosquitos.
Thanks for writing this post, I 100% share your sentiment and appreciate the depth with which you've explored this topic, including some of the political considerations.
Here are some other potentially-relevant case studies of people doing similar-ish things, trying to make the world a better place while navigating touchy political fears related to biotech:
- The "Enhanced Games" is organizing an alternative to the Olympic games where doping and other human enhancement technologies will be allowed. Naturally, they try to put a heavy emphasis on the importance of human freedom, plus some random criticisms of the way the existing Olympics are organized. But what I thought was especially striking was the way they leaned into social-justice-y rhetoric: "science is real", "stop exploitation", and even their logo is a dead ringer for the famous "equality" logo of the LGBT rights movement. For an antimalarial gene drive, I think a similar approach could work well (at least for winning the support of Westerners) -- leaning into rhetoric about postcolonialism and how the gene-drive initiative represents the people taking charge of their own destiny instead of waiting for western aid/charity (bednets, vaccines, etc) that hasn't been sufficient. (Charter Cities are in a somewhat similar situation, where it's very important for them to convince people that this is an empowering way of advancing human liberty while helping the developing world, rather than some kind of conniving neocolonialism intended to disempower people.)
- The C4 Rice Project has been operating for a long time, working towards the dream of engineering rice to more efficiently photosynthesize and thus boosting yields around the world.
- The Far-Out Initiative is hoping to trial gene editing to reduce animal suffering; their website has some interesting FAQs and in general the project has the same "activist genetics lab" vibe that a mosquito gene-drive lab might strive for.
- Same deal for the project to revive Woolly Mammoths -- the awesome documentary "We Are As Gods" is basically a PR campaign for the righteousness of this cause, and a good portrait of a similar movement which is farther along in the PR pipeline.
- Genomic embryo-selection companies like Lifeview and Orchid Health are also interesting in this context, although since they don't have to convince regulators or the wider public, they are keeping a lower profile for now. There are also some essentially stealth-mode groups who are investigating enhancements to the current state-of-the-art in embryo selection. These groups would be less interesting in terms of learning from their PR campaigns (they have none), but it might be helpful to study how they build a skilled team, raise funding, etc.
Some further questions I have about the political and theory-of-change considerations:
- I think it could be helpful to explore a more detailed breakdown of who exactly might be opposed, and for what reasons. And then try and figure out which of these sources actually matter the most / are the most real! For example:
- Maybe the people of a developing country will be opposed, because they just find GMOs scary / would be worried about being bitten by a GMO mosquito.
- Maybe neighboring countries will be opposed because they'll see it as an imposition on their sovereignty that an important decision (even if the decision is just... curing malaria, lol) is being taken without their input.
- Ordinary citizens and activists in the west might be opposed mostly because of some kind of "neocolonialism"/social-justice concerns, or mostly because of environmental concerns (removing mosquitoes might disrupt the environment), or mostly because of FDA-style biomedical caution and fear of GMOs.
- Elites in the developed world might be concerned from a perspective of international diplomacy and norms -- sure, THIS unilateral genetic engineering project will have amazing consequences, but will it end up net-negative if it encourages OTHER unilateral actions in the future that are more harmful? (Feels similar to the sentiment against climate geoengineering or human genetic editing.) What could be done to ameliorate this concern?
- Is there some way that a gene drive could be framed as partially accidental, or an inevitable by-product of some other necessary action? Sometimes you need a good excuse to do something good for the world... I am thinking of situations like:
- When an accidental freezer failure during covid vaccine distribution actually resulted in giving lots more people the vaccine, because it helped doctors get around onerous verification requirements about who was allowed to get one.
- Geoengineering experiments are still very taboo, but a recent UN regulation reducing sulfur dioxide emissions is unintentionally warming the earth and also giving scientists tons of data about the efficacy of future SO2-based climate interventions.
- Similarly, fertilizing the oceans with iron to fight climate change is considered taboo. But it might be easier to argue for fertilizing the oceans with iron in order to help sperm whale populations recover, since sperm whales once naturally helped iron cycle through the oceans but our own human actions caused their populations to decline. (Fighting climate change would thus just be an "accidental" benefit of helping the sperm whales.)
- In the malaria gene-drive case, the best possible headline is probably always gonna be "unanimous international agreement reached to release gene drive!" But as a second-best option, "truck carrying mosquitoes for gene-drive study crashes, thousands of GMO mosquitoes released, scientists very apologetic!" is DEFINITELY preferable to "rogue bio-activists publish manifesto, release thousands of GMO mosquitoes". And it might even be preferable to something like "president of Ghana, citing failure of western aid and the hypocrisy of colonial powers, unilaterally begins GMO drive".
- I'd also note that, although most prevalent in africa, malaria and other mosquito-borne diseases are common throughout the tropics. So although africa might be ideal from an impact standpoint, getting a project off the ground in southeast asia or central america is also worth considering, if the politics are more favorable / if it would be easier to set up a genetics lab there!
I like your idea of exploring "a more detailed breakdown of who exactly might be opposed, and for what reasons. And then try and figure out which of these sources actually matter the most / are the most real!"
It reminds me of "Is that your true rejection?" https://www.lesswrong.com/posts/TGux5Fhcd7GmTfNGC/is-that-your-true-rejection
>Same deal for the project to revive Woolly Mammoths -- the awesome documentary "We Are As Gods" is basically a PR campaign for the righteousness of this cause, and a good portrait of a similar movement which is farther along in the PR pipeline.
Unfortunately, on this one the hype has outpaced the science. See: https://www.lesswrong.com/posts/ihq24ri5g5svwwmjx/why-i-m-skeptical-of-de-extinction
The LessWrong Review runs every year to select the posts that have most stood the test of time. This post is not yet eligible for review, but will be at the end of 2024. The top fifty or so posts are featured prominently on the site throughout the year.
Hopefully, the review is better than karma at judging enduring value. If we have accurate prediction markets on the review results, maybe we can have better incentives on LessWrong today. Will this post make the top fifty?
Central America might work better than Africa. An interesting proposal I read, from Curtis Yarvin (framed as advice to the president of El Salvador to follow his extermination of the cartels) includes this as suggestion: https://graymirror.substack.com/p/salvador-as-a-startup-state.
I notice I feel some opposition to this, mostly on the grounds that messing with nature tends to end rather poorly for us. Nature is trillions of deeply interconnected dimensions doing who-knows-what at every layer; there is a small chance that release of these gene drives could be an x-risk. So do we take a guaranteed 600,000 dead every year, or an x% chance of accidentally wiping out all life on Earth? What value of x is acceptably low?
I notice I feel some opposition to this, mostly on the grounds that messing with nature tends to end rather poorly for us.
Human lifespan is a lot longer since we started messing with nature, it's on average going well for us.
A transposon that moves to a species that has no defenses against it can already create a gene drive that wipes out a species in a natural way.
In the wild, we have ecosystems made out of very many species so that eliminating a single one does not wipe out all life on earth.
>there is a small chance that release of these gene drives could be an x-risk
I disagree (or, at least, I believe that mosquitoes are more of an x-risk than gene drives, although both are extremely small)
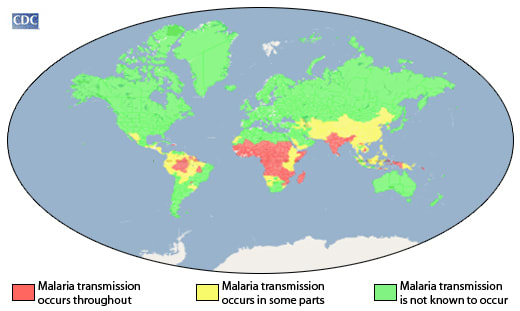
Last year, I wrote about the promise of gene drives to wipe out mosquito species and end malaria.
In the time since my previous writing, gene drives have still not been used in the wild, and over 600,000 people have died of malaria. Although there are promising new developments such as malaria vaccines, there have also been some pretty bad setbacks (such as mosquitoes and parasites developing resistance to commonly used chemicals), and malaria deaths have increased slightly from a few years ago. Recent news coverage[1] has highlighted that the fight against malaria has stalled, and even reversed in some areas. Clearly, scientists and public health workers are trying hard with the tools they have, but this effort is not enough.
Gene drives have the potential to end malaria. However, this potential will remain unrealized unless they are deployed – and every day we wait, more than 1,600 people (mostly African children) die. But who should deploy them?
I’m well aware of the Unilateralist’s Curse, and before publishing this post, I asked, why haven’t existing groups already released gene drive mosquitoes? Academic labs, and the nonprofit organization Target Malaria, have been working on gene drives for several years already, but they have been taking a cautious, incremental approach.[2] There are several good reasons for this:
Basically, this means that any organization releasing gene drives needs to ensure they are not banned. This would be difficult for a private organization, as influencing public opinion on GMOs is hard. However, if a government did this, it could simply choose not to ban gene drives (though it would need to remain firm against possible international pressure).
With that out of the way, I want to explore the technical details of gene drive construction, and describe what it would take for a moderately-well-equipped biology lab to build one targeting Anopheles gambiae. I emphasize that this is still a draft plan, and although I am skilled in molecular biology, I am not a mosquito specialist. But given the immense amount of death and suffering caused by malaria, I hope that the information I provide can help bring an end to this menace, by highlighting that political concerns, not technical challenges, are the main barriers to gene drive deployment.
How to make a gene drive
First, I want to thank Andrea Crisanti and his lab, not only for developing Anopheles-targeting gene drives, but also for making many of the technical details (including DNA sequences and mosquito editing methods) publicly available. This plan closely follows their strategy for making a gene drive.
There are three main steps: first, constructing the gene drive DNA; second, integrating it into mosquitoes; and third, releasing the mosquitoes into the wild.
The DNA
In 2020, the Crisanti lab reported a highly effective doublesex X-shredder gene drive, and helpfully, they published the DNA sequence for it. Note that it may be possible to make improvements on this gene drive,[4] but the overall process will be very similar regardless of which gene drive version is used.
The basic structure of the gene drive is shown in Figure 1 of the paper describing it:
The key functional parts are the SpCas9 nuclease driven by the germline-specific vas/zpg promoter,[5] the I-PpoI nuclease[6] driven by the male germline-specific Beta2 promoter,[7] and a guide RNA, driven by the constitutive U6 promoter. In the paper, the authors tested several versions of the drive, and found that one with a gRNA targeting the doublesex gene performed the best.[8] The drive version in the paper also contains fluorescent proteins to allow easy identification of drive-positive mosquitoes. However, these are functionally unnecessary, and a smaller gene drive payload will be more efficient, so it would be better to omit these.[9]
To integrate the gene drive DNA into mosquitoes, the Crisanti lab took a two-step approach. First, they performed a CRISPR knock-in of a small “landing pad” sequence containing attP recognition sites for φC31 integrase, as well as GFP under control of an eye-specific promoter,[10] into the doublesex gene. Next, they performed recombinase-mediated cassette exchange using a φC31 integrase plasmid and the gene drive plasmid, which contains attB sites that the φC31 enzyme recombines with the attP sites that were added in the first step. To follow this approach, we[11] will need four plasmids:
Because we know the sequences for all of these, we can order them from a commercial DNA synthesis provider such as Genscript, IDT, or Twist (all of which I’ve used for my own research). I got a quote from Genscript for $5600 to synthesize the smaller version of the gene drive plasmid, and $6500 for the larger version with the fluorescent proteins. If money is very tight, it would be cheaper to order the plasmid in smaller fragments and combine them using Gibson assembly. In this case, the DNA would cost approximately $900 for the small version and $1100 for the large version, although labor costs would be considerably higher.[13] In any case, assembling these plasmids, growing them in bacteria, and preparing DNA for injection will require only bachelor’s degree-level molecular biology work, one to two months of time, and at most $25,000 in DNA, reagents, and lab equipment.[14] Things will start getting more expensive in the next step.
The Mosquitoes
This project will require a mosquito breeding facility. If we have a permit, we can obtain Anopheles mosquitoes from a research repository, but I’m going to assume this will take place in an African country where we can catch mosquitoes from the wild as a backup option.[15]
For some weird reason,[16] the International Atomic Energy Agency publishes a 44-page guide on Anopheles mosquito husbandry. It covers quite a lot of details, including how to collect mosquitoes from the wild, how to build breeding cages, how to set up feeding stations (with sugar water, and cow blood for females after mating), and how to prevent common problems such as ant infestations. It also includes a list of references for more information on particular steps.
For even more detail, we can look at the comprehensive 408-page “Methods in Anopheles Research” published by the American Type Culture Collection and the NIH (chapters 1–4 are relevant for mosquito breeding, and it also covers mosquito microinjection). This also has a catalog of entomology suppliers, which will be very useful. The basic three-week breeding cycle is as follows:
I’m not going to do a detailed calculation of how much it would cost to build a mosquito breeding facility, but I’d roughly estimate somewhere around $100,000 in equipment (not counting the building itself), and two employees to run it. The time required to get it up and running would probably be around 6 months of intense work, which could be done in parallel with the plasmid preparations.
Editing and Release
Although setting up a breeding facility will be operationally difficult, the most technically challenging part of this whole project will be doing the actual mosquito editing. Fortunately, the Crisanti group published a helpful guide on how to inject DNA into mosquito eggs to perform editing. This is all explained pretty well in the guide (they even include catalog numbers of key equipment to buy), and I don’t have much to add here. The following equipment will be required (prices are from suppliers):
I’d estimate the total equipment cost to be roughly $28,000. After obtaining the equipment, the basic steps are to:
It will probably take a few months of practice to get right, and it would be a good idea to practice using a simple plasmid to make the embryos express GFP, before moving on to more complicated editing. We will need to do two rounds of editing: one to introduce the attP “landing pad” and another to swap out the “landing pad” for the gene drive. Then, we will need to breed gene drive mosquitoes. This could be a bit annoying due to the tendency of the gene drive to crash populations, so we will need a good supply of females to expand the numbers. Because of the PpoI nuclease X-shredder design, we will know our gene drive is working when all the larvae are males. Releasing a few thousand males would be sufficient to introduce the gene drive allele into the local population. Mosquitoes lay about 100 eggs each and the breeding cycle is 3 weeks long, so starting from a single edited mosquito it would take only a few months to breed the required numbers. I’d estimate another 6 months for this phase of the project if everything goes perfectly – which it probably won’t, so maybe 12 months is more realistic.
Post-Release Monitoring
After releasing the gene drive, we will need to check for resistance in order to develop updated drives to defeat the resistance. This will involve capturing mosquitoes from the wild (assuming there are any left), extracting their DNA, and sequencing it. PCR amplification of the target site, followed by Sanger sequencing, should be sufficient to test for resistant alleles at the target site at a cost of about $5 per sample. For larger numbers of samples, we could do the PCR in a barcoded format, pool samples, and do next-generation sequencing. This could scale very well (approximately 1 cent per sample, assuming batches of 250,000) but would require a larger upfront cost. Still, at this point the bigger cost would be collecting mosquitoes.
It’s also possible that resistance could arise due to mutations elsewhere in the genome, so if we observe that mosquito populations are rebounding, we should perform whole genome sequencing, at a cost of about $500 per sample.
Post-release monitoring may actually be the most expensive part of the project, because if the gene drive works, it will spread internationally, requiring collection of mosquitoes from a large area. Monitoring should continue until eradication is achieved, which may take a few years depending on mosquito migration rates.[20]
Public Relations
Since people will eventually find out about this project (even if it’s initially secret, they’ll notice when mosquitoes start disappearing), it’s best to take a proactive stance on public relations to mitigate backlash. This will be equally, if not more, important in comparison with the rest of the project, since we really want to avoid getting gene drives banned. Non-gene-drive transgenic mosquito technology has been accepted in several countries,[21] and an important part of this was convincing people of the benefits. We should emphasize the project’s potential to end the vast amount of death and suffering caused by malaria. As a top scientist of Target Malaria recently expressed in a news article:
A call for political action
Overall, I think this gene drive could be built and released for under a million dollars (considering equipment and salaries), although a budget of a few million would allow the project to move faster by hiring more people. Of course, the reason this is so cheap is that the R&D costs have already been paid by the Crisanti lab and others. I want to emphasize that releasing a gene drive, without having the R&D capacity to make a followup drive in the likely case that the first drive is insufficient, will be doing more harm than good. So, realistically this would be a multi-million dollar project.
But I also want to emphasize that this is well within the capabilities of most African governments! Just to pick a random sub-Saharan country, the national revenue of Ghana is about 65 billion Ghana Cedi, or $5.5 billion USD at October 2023 exchange rates.[22] Funded at $5 million per year, this would be about 1/1000 of total government revenue – and I’m sure the Ghanaian government could get some foreign support if it asked. For a larger country like Nigeria, it would be a trivial expense.
Thus, the barriers holding back gene drive deployment are political, not technical. A recent news article covering gene drives observed that:
So, assuming you’re not an African government official,[24] what can you do to help? I’m not a politician or PR expert, but here are some ideas:
Send an email to a Nigerian prince about your unique investment opportunityIdentify which African government officials have the power to approve gene drives in their countries, and encourage them to support this.If you have a typical reading speed,[26] in the time it’s taken you to read this far, thirteen more people have died of malaria. Gene drives can end this ongoing tragedy, but only if we decide to use them.
I thank Devon Stork for his helpful feedback on a draft of this article.
See: https://www.nytimes.com/2023/09/29/health/mosquitoes-malaria-disease-climate-change.html and https://www.nytimes.com/2023/09/29/health/mosquitoes-stephensi-malaria-africa.html
The last news from Target Malaria was in 2022, when they reported results of releasing non-gene-drive sterile male mosquitoes in Burkina Faso. As far as I know they have no active plans to release gene drives, not even limited “split drive” or “daisy drive” versions.
Even target sites with a low expected fitness of resistant alleles (such as doublesex) still have this possibility, and the mosquito population is very large.
Interestingly, a simulation paper found that a more basic version of the gene drive without the PpoI X-shredder may be better at spreading through heterogeneous populations. Basically, if the fitness reduction is too high, the drive allele dies out too quickly before spreading. But, the X-shredder drive has a lower chance of resistant alleles forming. As a reminder, the first gene drive release is unlikely to be completely successful, so it’s important to not have gene drives get banned!
Surprisingly, they seem to be using a version of Cas9 codon-optimized for human expression instead of insect expression.
This nuclease shreds the X chromosome, making all sperm male.
They found that a slightly modified promoter with lower activity performed better.
doublesex is a highly conserved gene involved in sex determination. When the intron 4–exon 5 splice site is disrupted, females cannot develop properly but males are fine. Because the sequence is so conserved, doublesex drives have a chance of spreading into other Anopheles species besides A. gambiae if hybridization takes place. This is probably a good thing, as those other Anopheles species also spread malaria.
Furthermore, one hypothetical resistance mechanism is mosquitoes evolving to not mate with fluorescent mosquitoes.
To make the version without fluorescent proteins, I downloaded their DNA file and opened it in Benchling. I highlighted the gene drive sequence in the screenshot below.
The rest of the sequence is for allowing the plasmid to be propagated in bacteria. At 14,465 base pairs, it’s a relatively big plasmid. I also designed a version of the plasmid with non-functional elements removed (including the fluorescent proteins), making the gene drive sequence about 20% smaller (the bacterial sequence is unchanged).
This is very helpful for identifying mosquitoes where the integration was successful. Also, at the second stage where the gene drive sequence is integrated into the doublesex site, loss of GFP indicates success.
I’m going to be using “we” here to mean the people building the gene drive, and this does not indicate that I will be personally involved.
Or possibly through modifying the gene drive plasmid, which already expresses Cas9 and an sgRNA targeting doublesex, to have the constitutive promoter from the φC31 plasmid driving Cas9 instead of the germline-specific zpg promoter.
If I did this personally, it would probably take me about a month of lab work. If I ordered the plasmids instead, it would take 2 days of hands-on work.
Centrifuges, incubators, etc. Note that it will be important to have 24-hour electricity which many African countries do not have, so expenses for a generator will also likely need to be added.
Doing this in an area where mosquitoes are endemic means that if any mosquitoes escape, they might spread the gene drive. This has generally been considered a bad thing, but if we’re planning to release them anyway, it’s not terrible – except for the PR problems it would cause.
It’s because radiation is used to sterilize mosquitoes for population suppression.
Alternatively we could buy a micropipette puller for about $5,000 and have a functionally unlimited supply.
A dissection microscope, pipettes, Petri dishes, filter paper, strainers, etc.
I learned how to do this on frog and mouse eggs at Frontiers in Reproduction. It took me a few days to learn, but I was helped by an experienced teacher.
Migration rates, not breeding rates, will be the limiting factor here. See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4306333/ and https://pubmed.ncbi.nlm.nih.gov/9439109/ for differing estimates.
To be specific, Oxitec’s sterile Aedes mosquitoes.
Although, the budget document indicates Ghana is at a high risk of default on its foreign loans. Still, the expenditure would be small compared to other government programs, and would have a high rate of return.
This project is funded by Open Philanthropy and intends to use a gene drive not to eradicate mosquitoes, but to make them express anti-malaria antibodies and not transmit malaria. However, a gene drive expert privately expressed various concerns to me about this project, such as that it will likely spread outside its intended area (the islands of São Tomé and Príncipe) to mainland Africa, and cause backlash in mainland countries leading to gene drives being banned. Furthermore, it may cause resistance in mosquitoes, or the evolution of malaria strains that are resistant to the anti-malaria antibodies. I hope the project can address these concerns before deploying their gene drive.
And if you are an African government official, I’d love to talk with you about this!
Although this is less likely to work than getting one country to allow gene drive release, it is still worth trying.
(2848 words, not counting footnotes) / (250 words/minute) * (600,000 deaths in last year) / (525600 minutes in last year) = 13 deaths